24/01/2025
Extraction of lithium from different feedstocks showing promising results
Partners working on various extraction processes of lithium (Li) from a variety of feedstocks – concentrates, waste cathode material, ore and tailings, are reaching target recovery rates.
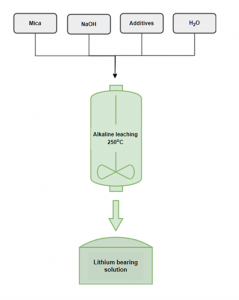
NTUA researchers have developed an alkaline leaching process to extract Li from spodumene concentrates, which yielded extraction rates of over 92 % and low impurities. The same process, this time applied on lithic mica, resulted in Li extraction rates of nearly 100 % at a longer leaching duration. Moreover, the optimal settings showed the capacity to maintain the level of impurities low. Leaching experiments on mica samples will continue, but results are already encouraging. This new leaching process requires temperatures considerably lower than the conventional extraction process, currently at 1100°C.
Alkaline leaching scheme to extract lithium NTUA
On their side, researchers at TEC have been optimising the solvometallurgical process to extract valuable elements from four type of materials: spodumene concentrates, lithic mica, lithium phosphate and off specification cathode material. After achieving their target of more than 95 % Li extraction from spodumene, the optimisation phase tested (taking advantage of the result from the novel pre-treatment established and previously described) milder leaching conditions, obtaining similar good results. For lithic mica and lithium phosphates, best operations routes investigated have shown that pre-treatment increases considerably the leaching yield at values higher than the target. For the off-specification cathode material, researchers have concluded that mechanical activation of the cake obtained after leaching improves Li extraction, achieving up to 99 % Li recovery and very high selectivity at room temperature processing. Ni, Co and Mn can be separated as a valuable mixture in the same process.
Finally, the research team at KIT, in charge of the reactive milling and aqueous leaching of waste cathode material [NMC], optimised the purification processes using various reducing agents. The intermediary results yielded Li recovery rates ranging between 68,8 % and 91 %, depending on the reducing agent utilised during the purification process. Next steps for KIT research group expand to calculating the lithium carbonate [Li2CO3] purity, determining the recovery rate of Ni, Mn and Co and upscaling the ball-milling.
Separation and purification of lithium from solutions
VITO researchers, working on the Li-sieve adsorption and desorption from aqueous leachates, shaped the lithium-titanium-oxide (LTO) adsorbents into spheres, which enabled dynamic testing. The optimised flow rates and settings initially modelled on synthetic Li solutions have been recently tested on real samples, yielding approx. 85 % Li recovery from aqueous alkaline spodumene leachates. The team at VITO has recently filed a patent application with the desorption stability results.
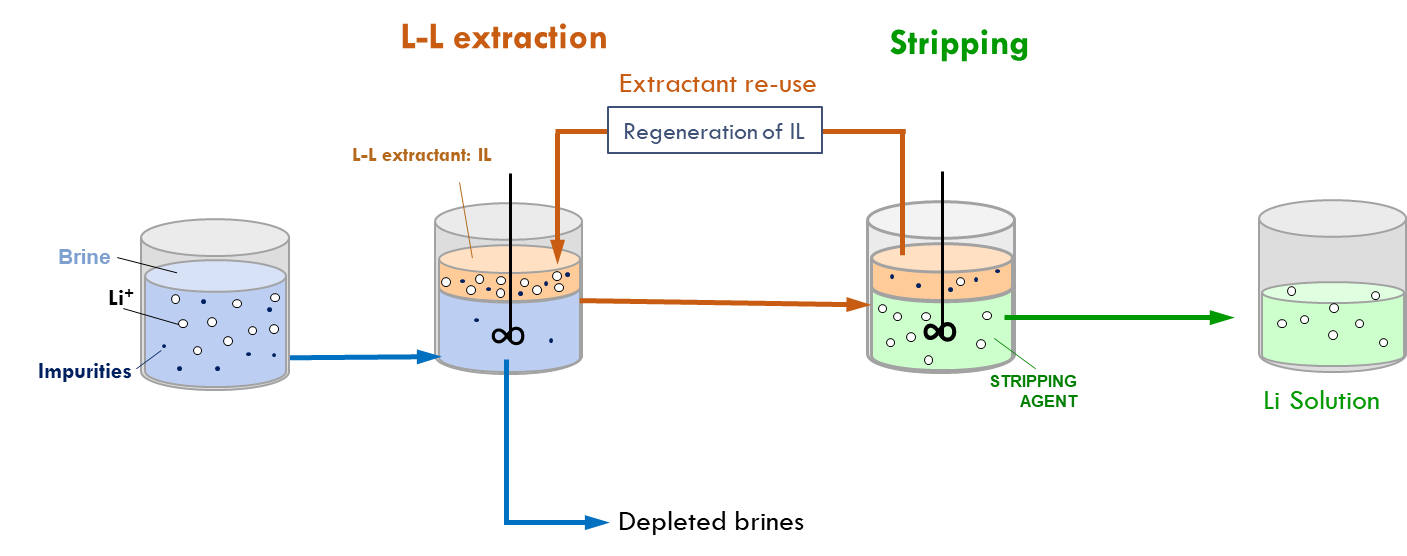
Expected results have already been shaping up in Spain, where TEC is working on the Li extraction from both continental and geothermal brines. After running tests using the most suitable extractants for their liquid-liquid extraction process [L-L] coupled with stripping operations, researchers have managed to obtain a global Li extraction of 92 % from continental brine, far beyond the initial target of 85 %, while diminishing the content of the accompanying cations (Na, K, Ca and Mg). On the other hand, the same technological process applied to spodumene yields a global recovery rate around >90 % after optimisation of the scenarios based on McCabe-Thiele diagrams.
In another European region, famous for its geothermal resources, EnBW researchers have been investigating Li-extraction from brines. They developed a novel synthesis route for Lithium Manganese Oxide [LMO] adsorbent, for which a patent has been recently filed. The LMO adsorbents have been demonstrating high absorption capacity and selectivity for Li extraction from brines with high salt contents. Offering improved chemical stability and potential for large-scale production of the material, this solution looks very promising for future implementation at industrial level for Li recovery.
Another extraction process, the electrode-based Li adsorption and desorption from brines, has been optimised by KIT. Following the principles of a salt water battery, the electrochemical extractions with Li-selective electrodes yielded encouraging results for the Li-extraction from geothermal brines. The Li selectivity in the recovery solution were in the range of 77 % to 82 %, displaying a good separation from the main contaminants.
© visual: TECNALIA