
Reactor used for the solvometallurgical leaching experiments by TEC
Various research partners involved in the LiCORNE project have been exploring different Li extraction technologies from Li-rich ores, tailings and off-specification cathode materials from battery production. All these exploratory routes, including alkaline leaching [NTUA], advanced solvometallurgy [TEC] and reactive ball-milling [KIT], share common objectives, aiming to be more energy efficient and reduce the environmental impact.
TEC’s advanced solvometallurgy approach leverages deep eutectic solvents to extract lithium, providing an energy-efficient solution for selective removal. This technique is not only applicable to Li but also extends to the extraction of other critical elements contained in the off-spec cathode materials.
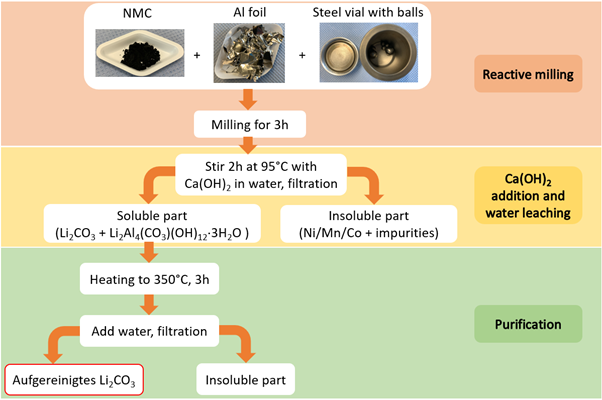
Meanwhile, KIT’s reactive ball-milling method is being explored as an effective battery recycling process. This innovative approach uses aluminium as a reducing agent for transition metals, which is already present in the input waste stream as the current collector material for electrodes. The process offers a direct route to battery-grade lithium carbonate.
TEC investigated and developed a solvometallurgical extraction process for lithium from spodumene concentrate, lithic mica and lithium phosphate, and for lithium, cobalt and nickel from off-specification cathode material. The optimised operating conditions and necessary pre-treatment steps enabled over 95% extraction of Li, Co and Ni from these materials at room temperature. Additionally, the reuse of the organic solvents utilised during the leaching processes was effectively tested proving that it does not affect the yield in the next cycles. The lithium containing liquid streams obtained are processed by TEC in further steps with different technologies towards the obtention of pure battery-grade lithium carbonate.

Reactor used for the solvometallurgical leaching experiments by TEC
Researchers at KIT studied in depth various ball-milling parameters for the mechanochemical transformation of the off-specification cathode material samples provided by Umicore. Subsequent water leaching facilitated the separation of an insoluble metallic composite containing Ni, Mn and Co from water soluble Li-compounds. KIT researchers optimised various reducing agents – such as Al, Ca and Mg, achieving Li recovery exceeding 80 %, with a Li2CO3 purity of around 90 %.
Product streams obtained by the various extraction technologies here explored will be further processed in subsequent separation and purification processes and lithium recovery methods. © KIT
Product streams obtained by the various extraction technologies here explored will be further processed in subsequent separation and purification processes and lithium recovery methods.
During conventional mineral processing, significant resources are often lost during the beneficiation phase. Lithium-bearing particles trapped in the gangue can proceed to downstream stages, reducing the efficiency of the entire extraction process. To address this, researchers at TU Delft have developed an Opto-Magnetic Sorting System that significantly enhances the separation of lithium ores. This innovative technology combines precision liquid deposition and magnetic separation techniques, offering an advanced alternative to traditional gravity-based separation methods used in beneficiation circuits.
The process starts with lithium-bearing ores being crushed and sieved, isolating particles in the 2–4 mm size range for the next step – optical sorting. A high-resolution line scan camera captures continuous images of particles on a conveyor belt. These images are processed in real-time using a custom algorithm developed at TU Delft, which is trained to identify lithium-rich particles based on subtle colour differences.
Once identified, the target particles are selectively marked using magnetic powder. This enables the marked lithium-rich particles to be separated efficiently by a downstream magnetic conveyor into a dedicated container.
This innovative beneficiation approach has successfully prevented around 45% of the gangue material from entering the downstream process—nearly three times more efficient than the initially targeted improvement of 15%.
According to the State-of-the-Art [SoA], processing spodumene takes place at high-temperatures [1100oC], with direct implications on the economic viability of the entire process. Researchers at TEC have been investigating an alternative to conventional processes. Their investigation features ball milling and calcination at lower temperatures than the conventional process, using additives when needed for the improvement of the next leaching step.
Ball milling is a mechanical process that induces self-sustaining reactions in many sufficiently exothermic powder mixtures. These exothermic reactions, which release a significant amount of heat, can influence both the microscopic and macroscopic properties of the resulting material. On a microscopic level, the heat generated by the reactions can cause changes in the crystal structure and composition of the material. On a macroscopic level, these changes can affect the material’s overall properties, such as its strength, hardness and reactivity. TECNALIA’s findings show that the combination of the ball milling with additives lower calcination temperatures required [200oC below the SoA] in the pre-treatment process of the samples and, also, allow milder conditions in the next processing phases (leaching).
The process, replicated on lithic mica and lithium phosphate materials, were also successful to achieve good results in the next leaching step.

The furnace used in the calcination pre-treatment by TECNALIA
Partners working on various extraction processes of lithium (Li) from a variety of feedstocks – concentrates, waste cathode material, ore and tailings, are reaching target recovery rates.
NTUA researchers have developed an alkaline leaching process to extract Li from spodumene concentrates, which yielded extraction rates of over 92 % and low impurities. The same process, this time applied on lithic mica, resulted in Li extraction rates of nearly 100 % at a longer leaching duration. Moreover, the optimal settings showed the capacity to maintain the level of impurities low. Leaching experiments on mica samples will continue, but results are already encouraging. This new leaching process requires temperatures considerably lower than the conventional extraction process, currently at 1100°C.
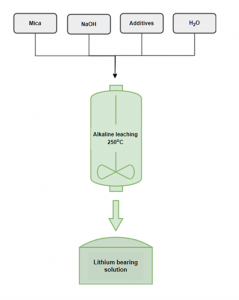
Alkaline leaching scheme to extract lithium NTUA
On their side, researchers at TEC have been optimising the solvometallurgical process to extract valuable elements from four type of materials: spodumene concentrates, lithic mica, lithium phosphate and off specification cathode material. After achieving their target of more than 95 % Li extraction from spodumene, the optimisation phase tested (taking advantage of the result from the novel pre-treatment established and previously described) milder leaching conditions, obtaining similar good results. For lithic mica and lithium phosphates, best operations routes investigated have shown that pre-treatment increases considerably the leaching yield at values higher than the target. For the off-specification cathode material, researchers have concluded that mechanical activation of the cake obtained after leaching improves Li extraction, achieving up to 99 % Li recovery and very high selectivity at room temperature processing. Ni, Co and Mn can be separated as a valuable mixture in the same process.
Finally, the research team at KIT, in charge of the reactive milling and aqueous leaching of waste cathode material [NMC], optimised the purification processes using various reducing agents. The intermediary results yielded Li recovery rates ranging between 68,8 % and 91 %, depending on the reducing agent utilised during the purification process. Next steps for KIT research group expand to calculating the lithium carbonate [Li2CO3] purity, determining the recovery rate of Ni, Mn and Co and upscaling the ball-milling.
VITO researchers, working on the Li-sieve adsorption and desorption from aqueous leachates, shaped the lithium-titanium-oxide (LTO) adsorbents into spheres, which enabled dynamic testing. The optimised flow rates and settings initially modelled on synthetic Li solutions have been recently tested on real samples, yielding approx. 85 % Li recovery from aqueous alkaline spodumene leachates. The team at VITO has recently filed a patent application with the desorption stability results.
Expected results have already been shaping up in Spain, where TEC is working on the Li extraction from both continental and geothermal brines. After running tests using the most suitable extractants for their liquid-liquid extraction process [L-L] coupled with stripping operations, researchers have managed to obtain a global Li extraction of 92 % from continental brine, far beyond the initial target of 85 %, while diminishing the content of the accompanying cations (Na, K, Ca and Mg). On the other hand, the same technological process applied to spodumene yields a global recovery rate around >90 % after optimisation of the scenarios based on McCabe-Thiele diagrams.
In another European region, famous for its geothermal resources, EnBW researchers have been investigating Li-extraction from brines. They developed a novel synthesis route for Lithium Manganese Oxide [LMO] adsorbent, for which a patent has been recently filed. The LMO adsorbents have been demonstrating high absorption capacity and selectivity for Li extraction from brines with high salt contents. Offering improved chemical stability and potential for large-scale production of the material, this solution looks very promising for future implementation at industrial level for Li recovery.
Another extraction process, the electrode-based Li adsorption and desorption from brines, has been optimised by KIT. Following the principles of a salt water battery, the electrochemical extractions with Li-selective electrodes yielded encouraging results for the Li-extraction from geothermal brines. The Li selectivity in the recovery solution were in the range of 77 % to 82 %, displaying a good separation from the main contaminants.
© visual: TECNALIA
Within the beneficiation process, the research group at TUD developed an opto-magnetically induced sorting technology. Within the next months, their work will continue developing their code to optimise the colour identification of target metals, and simultaneously on various set-ups to improve the magnetic attraction and and to ensure the seamless integration of all components of their opto-magnetically induced sorter.
Within the same work package, researchers at NTUA have developed a new calcination technology with additives, tested on spodumene concentrates. Using different settings and parameters, such as the processing temperature, reaction time, pressure, the extraction yields for Li ranged between 71 % and 96 %. Depending on the additive type, adjusting the calcination parameters accordingly can significantly reduce impurities, such as aluminum (Al), present in spodumene concentrate. Simultaneously, NTUA partners have been optimising a new technology for Li extraction with calcination from lithic mica and the results will be available in the upcoming communications.
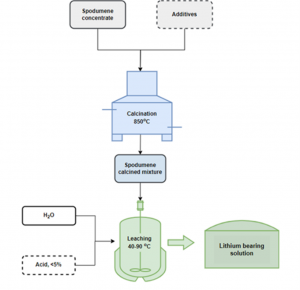
Calcination scheme NTUA
Working on spodumene concentrates, the research group at TEC has established a novel pre-treatment process that allows a relevant improvement in the next leaching process of lithium for its valorisation. As a result of this method, which includes ball milling combined with additive, the transformation of the mineralogical structure of the spodumene takes place at a significantly reduced temperature, ranging from 1100ºC to 900ºC. Based on these findings, TEC has applied a similar approach for the lithium phosphate and the lithic mica materials, reporting good results.
© visual:Adobe Stock Photos
On 16 October 2024, the Karlsruhe Institute of Technology (KIT) was hosting not only the LiCORNE project’s M24 consortium meeting, but also its first exploitation workshop. The event brought together a diverse group of stakeholders, with nearly 15 industry guests and members of the External Advisory Board (EAB), to discuss the latest advancements in lithium (Li) extraction technologies.
The workshop began with a welcome address by Dr. Lourdes Yurramendi [the coordinator of the LiCORNE initiative and Project Director at TECNALIA Waste Valorisation, Energy, Climate and Urban Transition], followed by Nader Akil, Operations Manager at PNO Innovation Belgium, who outlines the objectives of the exploitation workshop and provided an overview of the LiCORNE project. Funded by the European Commission, the project aims to develop competitive technologies for Li extraction and recovery from various feedstocks, including ores, geothermal brines and cathode waste materials. Following this introduction, various partners delivered technical presentations, showcasing their innovative approaches and key exploitable results after 24 months from the start of the project.
Regardless the feedstock considered, all these novel technologies share one theme: sustainability. This focus on sustainability translates into exploring research routes that go beyond the current state-of-the-art (SoA), reducing energy and water consumption and the generation of chemical waste:
Beyond technological presentations, the workshop also facilitated discussions with external participants, including members of the EAB and industry representatives. These exchanges provided valuable insights into the industry’s needs and opened up new routes for collaboration. To facilitate future collaborations, PNO presented several funding opportunities that can be used to bring the most promising technologies and the LiCORNE selected flowsheet to a pilot level.
As the project progresses, the focus will shift now towards the benchmarking and selection of the most promising LiCORNE technologies for upscaling to produce ~1 kg of battery-grade Li by the end of the project. This phase aims to shape a path towards larger piloting and future commercialisation.
Using alkaline leaching process on spodumene concentrate, the maximum extraction of Li achieved thus far reached 75%. The leachate transformation, even after the filtration step, hinders the sample analysis and further processing. To overcome this challenge, upcoming experiments will explore elevated temperatures, diverse additives, and further investigate the chemical precipitation process.
During the advanced solvometallurgy applied on spodumene concentrate, the research team at TECNALIA reported high Li leaching yields (>95%). Their future work will focus on the further optimisation of the operational conditions, more appropriate for the anticipated scalability phases of the process. On the other hand, solvometallurgical tests carried on waste cathode material achieved high leaching yields for all target elements (Li, Co, Ni, Mn) using mild operational parameters.
After the first experiments engaging reactive milling and aqueous leaching [treated with aluminium- (Al) and calcium (Ca) – salts] on waste cathode material, researchers at KIT reported close to 31% Li recovery rate. Samples supplied by UMICORE were leached under different conditions to extract Li – available in the form of Li carbonate [LiCO3], and further subjected to purifications processes employing various reducing agents. Future efforts for this particular task will focus on adjusting leaching temperatures, establishing an optimal purification process, and evaluating Li recoverability in both Al and Ca systems.
Anticipating future upscaling phases, researchers at VITO, working on the Li-sieve adsorption and desorption from aqueous leachates, shaped the lithium-titanium-oxide (LTO) adsorbents into spheres, which enabled dynamic testing. The team is currently optimising the flow rates for adsorption and desorption to model the optimal conditions for upcoming processes. While initial tests utilised synthetic Li solutions, upcoming research will extend to purification processes for spodumene leachates.
In the same work package, TECNALIA performed experiments using different organic solvents for the liquid/liquid (L/L) extraction from brines showing promising Li yields in the range of 40-60 %.
Within the same work package, EnBW scientific team has been working on designing an eco-friendly Li-desorption process from brines, focusing on the development of novel synthesis for Mn-based adsorbent material. Notably, the successful upscaling of the synthesis process from 2,5g to 200g marks a significant achievement in sustainable material synthesis.
Finally, the last task of WP5 – Electrode-based Li adsorption and desorption from brines, conducted by KIT, presented the conclusions of their research work carried during the last six months, which includes a 4-step process. Their work has been focusing recently on the optimisation of the electrode pre-treatment, the establishment of the current densities and the reduction of the Na contaminations. Despite high Li selectivity rates obtained thus far, their work in the upcoming months will centre around optimising the recovery efficiency and the selectivity. Future experiments will test different thermal operating conditions (40°, 60°, 80°), but will also attempt to scale-up the process.
In the final technical work package, SINTEF scientists are pioneering a two-step process which involves in a primary phase selective chlorination by converting insoluble oxides to soluble chlorides; this is followed by a second step – electrolysis of the soluble chlorides extracting the target elements. After conducting different chlorination experiments, researchers emphasised the importance of time and the processing duration, confirming over 65 % Li recovery rate. With promising results, their focus pivots towards the second step – electrolysis.
Read the next article for a comprehensive overview of the meeting.
Focusing on mechanochemical (MC) processing, the recovery of high-value components from the cathode waste supplied by UMICORE is planned to be performed within Task 4.3.
The ball milling process of waste cathode material was optimised at laboratory scale using different reducing agents such as Al, Ca, and their mixtures. The role of the MC conditions (ball-to-sample ratio (B/S), ball milling time, and nature of the reducing material) was further investigated and analysed. This showed the kinetics of the MC-induced reduction reaction is sensitive to multiple processing parameters.
After the reduction reaction, the powder X-ray diffraction (XRD) analysis revealed the formation of metallic composites and Al/or Ca oxides, as illustrated in the figure below. The upcoming research will be dedicated to investigating and optimising the aqueous leaching conditions of the ball-milled samples at laboratory scale.
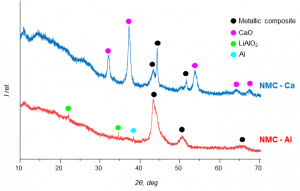
XRD patterns of the MC processed cathode waste materials with Al and Ca as reducing agents